Introduction:
Hemophilia, a congenital bleeding disorder resulting from clotting factor deficiency, significantly impacts the lives of persons due to recurrent bleeding. Prophylaxis, involving clotting factor replacement, has been recognized as a vital approach to reduce complications and to improve the quality of life in people with hemophilia. However, access to this remains limited, in low-income countries like India. This study examines the effectiveness of personalized low-dose clotting factor concentrates (CFC) prophylaxis and Emicizumab in children with hemophilia, aiming to achieve zero annual bleed rates (ABR), thereby enhancing their well-being and reducing healthcare burden.
Patients and Methods:
This retrospective observational study was conducted at the Hemophilia Treatment Centre (HTC) in Aluva, Kerala, India, spanning from May 31, 2022, to June 1, 2023. Study included children under 18 years with hemophilia A/B, with or without inhibitors, who had commenced prophylaxis with personalized low-dose CFC since 2015 and completed one year of prophylaxis. The total sample size was 31. Data on ABR, Annual Joint Bleed Rate (AJBR), Hemophilia Joint Health Status (HJHS) score, and Functional Independence Score Hemophilia (FISH) were extracted from the HTC registry.Standard half-life CFCs were used for prophylaxis, with a starting frequency of twice a week for Factor VIII and once a week for Factor IX. Patients were regularly followed up in the Hemophilia Clinic, and the frequency/doses of prophylaxis were increased in case of breakthrough bleeds. Additionally, children were advised to organize peak activities to coincide with the days of CFC injections. Personalization of doses was not based on pharmacokinetic software but was determined through close monitoring and individual adjustments.Health education on identifying and managing bleeding episodes followed ISTH guidelines. Prophylaxis outcomes were assessed with changes in ABR and AJBR during the last year of prophylaxis and compared to the bleed rates in the one year prior to prophylaxis. Changes in HJHS and FISH were documented for older children. All analysis were done using SPSS version24. For quantitative variables results were presented as mean with standard deviation and for qualitative variables, results were presented in percentages. Comparison achieved with standard paired t-test, and p value < 0.05 considered as significant.
Results:
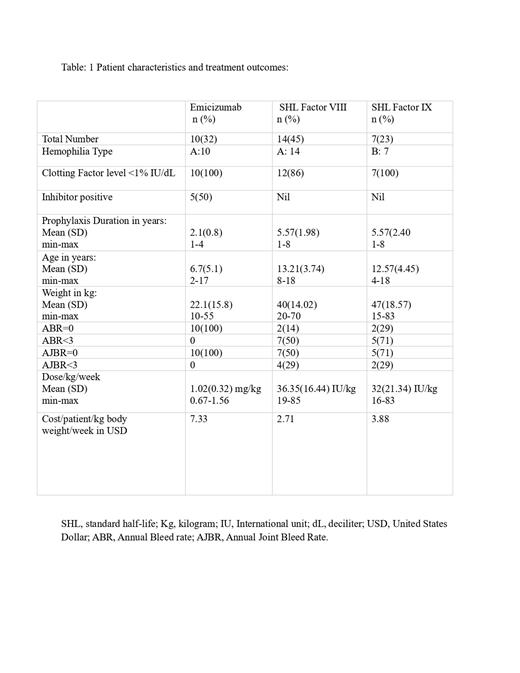
Out of 36 children receiving prophylaxis, 31 met the inclusion criteria. Among them,10 (32%) received Emicizumab prophylaxis, while 21 (68%) were managed with personalized low-dose CFC prophylaxis. Hemophilia A constituted 24(77%) of cases, mostly classified as severe disease-29 (94%). Table :1 summarises the patient characteristics, prophylaxis dose, cost and outcome measures of the study population.The personalized low-dose CFC prophylaxis had significant measurable improvements, reducing the ABR from 14.90 to 2.90( p 0.000) and the AJBR from 4.95 to 1.43( p 0.004). Additionally, HJHS improved from 3.30 to 0.95( p 0.102), and FISH increased from 29.90 to 32.00( p 0.020).Children on Emicizumab achieved exceptional results, with all reporting zero ABR ( p 0.027), and AJBR ( p 0.131) even with lower than recommended doses(1.02mg/kg/week). In contrast, personalized low-dose CFC prophylaxis (Factor VIII: 36.35/kg/week, Factor IX:32/kg/week) showed varied outcomes, with only 4(19%) achieving zero ABR. HJHS and FISH were not evaluated in Emicizumab group as 7(70%) were belonging to the age group of less than 5 years.
Conclusion:
This study highlights the effectiveness of personalized low-dose CFC prophylaxis and Emicizumab in reducing bleeding rates and improving musculoskeletal health and functional independence in children with hemophilia. Emicizumab prophylaxis demonstrated remarkable outcomes, achieving zero bleeds even with lower doses. However, personalized low-dose CFC prophylaxis struggled to achieve zero bleed rates for most of them. The findings underscore the need for alternative strategies and increased accessibility to Emicizumab and other newer drugs to achieve the goal of zero bleed rates for children with hemophilia in resource-limited settings. The study's results provide hope for achieving zero bleed rates, ultimately enhancing the well-being of children with hemophilia in resource-limited regions.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal